when it comes to bonding with other atoms, what is phosphorus most likely to do?
The Incomplete Octet
While most elements below atomic number 20 follow the octet rule, several exceptions exist, including compounds of boron and aluminum.
Learning Objectives
Describe the ways that B, Al, Li, and H deviate from the octet dominion
Key Takeaways
Key Points
- The octet dominion states that atoms with an diminutive number below 20 tend to combine so that they each have eight electrons in their valence shells, which gives them the same electronic configuration equally a noble gas.
- The two elements that almost commonly fail to complete an octet are boron and aluminum; they both readily form compounds in which they have 6 valence electrons, rather than the usual eight predicted by the octet rule.
- While molecules exist that contain atoms with fewer than eight valence electrons, these compounds are often reactive and tin react to form species with eight valence electrons. For example, BFthree will readily bind a fluoride anion to form the BF4 – anion, in which boron follows the octet dominion.
Key Terms
- atomic number: The number, equal to the number of protons in an atom, that determines its chemical properties. Symbol: Z.
- valence electrons: The electrons in the outermost (valence) principal energy level of an atom that tin can participate in the formation of chemic bonds with other atoms.
- octet rule: Atoms gain, lose, or share electrons with other atoms in order to fill their valence level with eight electrons.
The Octet Rule and Its Exceptions
The octet rule states that atoms below atomic number 20 tend to combine so that they each accept eight electrons in their valence shells, which gives them the aforementioned electronic configuration as a element of group 0. The dominion is applicative to the principal- group elements, especially carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium and magnesium.
Valence electrons can be counted using a Lewis electron dot diagram. In carbon dioxide, for instance, each oxygen shares four electrons with the central carbon. These 4 electrons are counted in both the carbon octet and the oxygen octet considering they are shared.
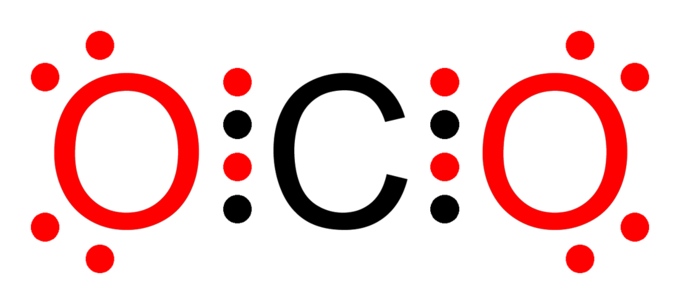
Carbon dioxide: A Lewis dot diagram for carbon dioxide.
Hydrogen and Lithium
However, many atoms below atomic number 20 often form compounds that do non follow the octet rule. For example, with the duet rule of the showtime principal energy level, the element of group 0 helium, He, has 2 electrons in its outer level. Since at that place is no 1p subshell, 1s is followed immediately by 2s, and thus level 1 can only accept at almost two valence electrons. Hydrogen only needs one boosted electron to attain this stable configuration, through either covalent sharing of electrons or by becoming the hydride ion (:H–), while lithium needs to lose one past combining ionically with other elements. This leads to hydrogen and lithium both having two electrons in their valence shell—the aforementioned electronic configuration every bit helium—when they form molecules by bonding to other elements.
Boron and Aluminum
There are also a variety of molecules in which in that location are too few electrons to provide an octet for every atom. Boron and aluminum, from Group III (or 13), display different bonding behavior than previously discussed. These atoms each have iii valence electrons, so we would predict that these atoms want to bond covalently in order to gain five electrons (through sharing) to fulfill the octet dominion. However, compounds in which boron or aluminum atoms form v bonds are never observed, so nosotros must conclude that simple predictions based on the octet rule are not reliable for Group Iii.
Consider boron trifluoride (BF3). The bonding is relatively simple to model with a Lewis structure if we let each valence level electron in the boron atom to exist shared in a covalent bond with each fluorine atom. In this compound, the boron atom merely has six valence shell electrons, merely the octet rule is satisfied by the fluorine atoms.

Lewis structure of boron trifluoride: Each pair of dots represents a pair of electrons. When placed between ii atoms, the electrons are in a bond. A bail can be drawn equally a line between 2 atoms, which besides indicates two electrons. Notice that the central boron atom has only 6 electrons in the terminal Lewis diagram/structure of this molecule.
We might conclude from this one example that boron atoms obey a sextet rule. However, boron will grade a stable ion with hydrogen, BH4 –, in which the boron atom does have a consummate octet. In addition, BFiii volition react with ammonia (NHiii), to grade a stable compound, NH3BF3, for which a Lewis structure can be drawn that shows boron with a complete octet.

Boron trifluoride-ammonia complex: This covalent compound (NH3BF3) shows that boron can have an octet of electrons in its valence level.
Compounds of aluminum follow similar trends. Aluminum trichloride (AlCl3), aluminum hydride (AlH3), and aluminum hydroxide (Al(OH)three) indicate a valence of three for aluminum, with 6 valence electrons in the bonded molecule. However, the stability of aluminum hydride ions (AlH4 –) indicates that Al can besides back up an octet of valence shell electrons.
Although the octet rule can still be of some utility in agreement the chemistry of boron and aluminum, the compounds of these elements are harder to predict than for other elements.
Odd-Electron Molecules
Molecules with an odd number of electrons disobey the octet dominion.
Learning Objectives
Describe the departure from the octet dominion by free radicals
Fundamental Takeaways
Cardinal Points
- While the majority of compounds formed from atoms below diminutive number 20 follow the octet rule, there are many examples of compounds that do non.
- Having an odd number of electrons in a molecule guarantees that it does non follow the octet rule, because the rule requires eight electrons (or two for hydrogen) effectually each atom.
- The most commonly encountered stable species that exist with an odd number of electrons are nitrogen oxides, such as nitric oxide (NO) and nitrogen dioxide (NO2), both of which are free radicals and disobey the octet rule.
Key Terms
- metastable: Of or pertaining to a physical or chemical country that is relatively long-lived, but may disuse to a lower energy state when perturbed.
- free radical: Whatsoever molecule, ion, or atom with one or more unpaired electrons. They vary in reactivity and stability from highly reactive, occurring as transient (curt-lived) species, to metastable.
- octet dominion: Atoms lose, gain, or share electrons in order to accept a full valence beat of eight electrons. Hydrogen is an exception because information technology can hold a maximum of two electrons in its valence level.
Free Radicals
Some elements, nigh notably nitrogen, can form compounds that do not obey the octet rule. One form of such compounds are those that accept an odd number of electrons. As the octet rule requires eight electrons around each atom, a molecule with an odd number of electrons must disobey the octet rule. Molecules with unpaired electrons are termed 'free radicals.' While typically highly unstable, and therefore highly reactive, some free radicals exhibit stability of days, months, or even years. These latter compounds are said to be ' metastable,' meaning they will decompose or react if given plenty fourth dimension, but are stable plenty for a considerable amount of time, from days to even years, when subjected to only minor disturbances.
Examples of Free Radical Molecules
Recall that the Lewis structure of a molecule must draw the total number of valence electrons from all the atoms which are bonded together.
Nitric oxide has the formula NO. The total number of valence electrons is 5+half dozen=11. Therefore, no matter how electrons are shared between the nitrogen and oxygen atoms, there is no fashion for nitrogen to have an octet. Information technology will have seven electrons, assuming that the oxygen atom does satisfy the octet.

Nitric oxide: Nitric oxide (NO) is an instance of a stable free radical. It does not obey the octet dominion on the nitrogen atom. Each line around the atoms represents a pair of electrons.
Nitric oxide is a past-product of combustion reactions that occur in engines, similar those in automobile engines and fossil fuel power plants. It is besides produced naturally during the electrical discharge of lightning during thunderstorms.
Nitrogen dioxide is the chemical compound with the formula NO2. Over again, nitrogen dioxide does not follow the octet rule for one of its atoms, namely nitrogen. The total number of valence electrons is 5+2(half dozen)=17. At that place is persistent radical character on nitrogen because information technology has an unpaired electron. The ii oxygen atoms in this molecule follow the octet rule.

Nitrogen dioxide: Nitrogen dioxide is some other stable molecule that disobeys the octet rule. Notation the seven electrons around nitrogen. Formal charges and the molecule's resonance structures are indicated.
Nitrogen dioxide is an intermediate in the industrial synthesis of nitric acid, millions of tons of which is produced each year. This reddish-brown toxic gas has a characteristic sharp, bitter odor and is a prominent air pollutant.
The Expanded Octet
Chief group elements in the third period and below form compounds that deviate from the octet rule past having more than eight valence electrons.
Learning Objectives
Explain why some elements can form an expanded octet
Key Takeaways
Fundamental Points
- Chief grouping elements that form more than bonds than would be predicted by the octet dominion are called hypervalent compounds, and have what is known as an ' expanded octet,' meaning that there are more viii electrons effectually 1 atom.
- The octet rule can be 'expanded' past some elements by utilizing the d- orbitals constitute in the tertiary primary energy level and beyond. Sulfur, phosphorus, silicon, and chlorine are common examples of elements that form an expanded octet.
- Phosphorus pentachloride (PClfive) and sulfur hexafluoride (SF6) are examples of molecules that deviate from the octet rule by having more than eight electrons around the central cantlet.
Primal Terms
- main grouping element: Elements that are not part of the transition element block in the periodic table.
- expanded octet: A example where an cantlet shares more than 8 electrons with its bonding partners.
- hypervalent molecule: A molecule that contains an atom from a main group element which deviates from the octet rule past sharing more eight electrons.
Deviations from the Octet Rule
A hypervalent molecule is a molecule that contains one or more main group elements that bear more than 8 electrons in their valence levels as a consequence of bonding. Phosphorus pentachloride (PCl5), sulfur hexafluoride (SF6), chlorine trifluoride (ClFthree), and the triiodide ion (I3 −) are examples of hypervalent molecules.
For the elements in the second menses of the periodic table (principal free energy level n=two), the stwop6 electrons comprise the octet, and no d sublevel exists. As a result, the second menstruum elements (more than specifically, the nonmetals C, Northward, O, F) obey the octet rule without exceptions.

Phosphorus pentachloride: In the PCl5 molecule, the fundamental phosphorus atom is bonded to five Cl atoms, thus having 10 bonding electrons and violating the octet rule. The overall geometry of the molecule is depicted (trigonal bipyramidal), and bond angles and lengths are highlighted.
However, some of the 3rd-menstruation elements (Si, P, S, and Cl) have been observed to bond to more than four other atoms, and thus need to involve more the four pairs of electrons bachelor in an s2psix octet. This is possible considering for n=3, the d sublevel exists, and it has 5 d orbitals. Although the energy of empty 3d-orbitals is ordinarily higher than that of the 4s orbital, that difference is small and the additional d orbitals tin can accommodate more electrons. Therefore, the d orbitals participate in bonding with other atoms and an expanded octet is produced. Examples of molecules in which a third period cardinal atom contains an expanded octet are the phosphorus pentahalides and sulfur hexafluoride.

Sulfur hexafluoride: In the SFvi molecule, the cardinal sulfur atom is bonded to half dozen fluorine atoms, so sulfur has 12 bonding electrons effectually it. The overall geometry of the molecule is depicted (tetragonal bipyramidal, or octahedral), and bond angles and lengths are highlighted.
For atoms in the fourth period and across, higher d orbitals can exist used to accommodate additional shared pairs across the octet. The relative energies of the different kinds of atomic orbital reveal that energy gaps go smaller as the chief energy level quantum number (n) increases, and the energetic toll of using these higher orbitals to adapt bonding electrons becomes smaller.
Source: https://courses.lumenlearning.com/boundless-chemistry/chapter/exceptions-to-the-octet-rule/
0 Response to "when it comes to bonding with other atoms, what is phosphorus most likely to do?"
Post a Comment